Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal gammopathy and Skin abnormalities (POEMS) Syndrome in a Filipino Nurse Returning to Work Following Bortezomib Treatment: A Case Report
Nadine J. Endaya, Raymond L. Rosales
Oct 2022 DOI 10.35460/2546-1621.2022-0044 Access
Abstract
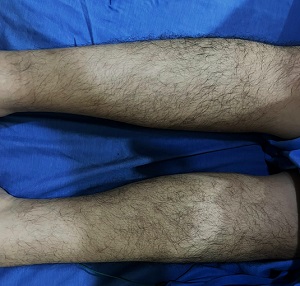
POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, M-protein, skin changes) also known as Crow-Fukase syndrome or Takatsuki syndrome is a rare and disabling paraneoplastic syndrome that frequently occurs in the fifth or sixth decade of life without a known standard first-line therapy. A 34-year-old Filipino male nurse, who presented with gradually progressive distal lower extremity weakness and sharp shooting pain in bilateral legs accompanied by mild joint effusion on the left knee, hypertrichosis, bilateral conjunctival injection, and gynecomastia underwent extensive workup and was diagnosed with POEMS syndrome. Complete blood count revealed erythrocytosis and thrombocytosis with elevated serum VEGF (vascular endothelial growth factor) and elevated monoclonal serum free lambda light chains. The electrophysiologic studies revealed chronic demyelinating sensorimotor polyneuropathy while bone marrow core biopsy and bone marrow aspirate smear immunohistochemical staining showed it to be positive for lambda and CD138. He had an initial unsuccessful treatment course with melphalan and prednisone. Hence, bortezomib and dexamethasone were given which gave significant improvement in symptoms from the overall neuropathy limitation score of 5 to 1.
Key words: POEMS syndrome, Crow-Fukase syndrome, Takatsuki syndrome, Bortezomib, Dexamethasone.
- Prado MB, Adiao KJ. Clinical features of POEMS syndrome in Southeast Asia: A literature-based study. SN Compr. Clin. Med. 2022;4(48). [cited 2022 June 18]. Available from: https://doi.org/10.1007/s42399-022-01130-3.
- Jurczyszyn A, Castillo JJ, Olszewska-Szopa M, Kumar L, Thibaud S, Richter J, et al. POEMS syndrome: Real world experience in diagnosis and systemic therapy – 108 patients multicenter analysis. Clinical Lymphoma, Myeloma & Leukemia. 2022; 22(5):279–304. [cited 2022 June 18]. Available from: https://doi.org/10.1016/j.clml.2021.10.007.
- Khwaja J, Keh R, Smyth D, Lunn MP, D’Sa S, Sive J. Daratumumab-bortezomib-dexamethasone use in relapsed POEMS syndrome. EJHaem [Internet]. 2022;3(3):1021–4. Available from: http://dx.doi.org/10.1002/jha2.492.
- He H, Fu W, Du J, Jiang H, Hou J. Successful treatment of newly diagnosed POEMS syndrome with reduced-dose bortezomib based regimen. Br J Haematol [Internet]. 2018;181(1):126–8. Available from: http://dx.doi.org/10.1111/bjh.14497.
- Nozza A. Poems Syndrome: An update. Mediterr J Hematol Infect Dis. 2017;9(1). 10.4084/MJHID.2017.051. [cited 2022 June 27].
- Misawa S, Sato Y, Katayama K, Hanaoka H, Sawai S, Beppu M, et al. Vascular endothelial growth factor as a predictive marker for POEMS syndrome treatment response: retrospective cohort study. BMJ Open [Internet]. 2015;5(11):e009157. Available from: http://dx.doi.org/10.1136/bmjopen-2015-009157
- Straume O, Bergheim J, Ernst P. Bevacizumab therapy for POEMS syndrome. Blood. 2006;107(12):4972–4. doi:10.1182/blood-2005-12-5045
- Ji ZF, Zhang DY, Weng SQ, Shen XZ, Liu HY, Dong L. POEMS syndrome: A report of 14 cases and review of the literature. ISRN Gastroenterol [Internet]. 2012;2012:584287. Available from: http://dx.doi.org/10.5402/2012/584287
- Dispenzieri A. POEMS syndrome: 2021 Update on diagnosis, risk- stratification and management. Annual Clinical Updates in Hematological Malignancies. 2021. Available from: https://doi.org/10.1002/ajh.26240.
- Ria R, Melaccio A, Racanelli V, Vacca A. Anti-VEGF drugs in the treatment of multiple myeloma patients. J Clin Med [Internet]. 2020;9(6):1765. Available from: http://dx.doi.org/10.3390/jcm9061765.
Articles related to the one you are viewing
There are currently no results to show, please try again later
![]() Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0
International License, which permits use, share — copy and redistribute the material in any medium or format,
adapt — remix, transform, and build upon the material, as long as you give appropriate credit,
provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner,
but not in any way that suggests the licensor endorses you or your use. You may not use the material for
commercial purposes. If you remix, transform, or build upon the material, you must distribute your
contributions under the same license as the original. You may not apply legal terms or technological
measures that legally restrict others from doing anything the license permits. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons
license and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this license,
visit https://creativecommons.org/licenses/by-nc-sa/4.0/.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0
International License, which permits use, share — copy and redistribute the material in any medium or format,
adapt — remix, transform, and build upon the material, as long as you give appropriate credit,
provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner,
but not in any way that suggests the licensor endorses you or your use. You may not use the material for
commercial purposes. If you remix, transform, or build upon the material, you must distribute your
contributions under the same license as the original. You may not apply legal terms or technological
measures that legally restrict others from doing anything the license permits. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons
license and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this license,
visit https://creativecommons.org/licenses/by-nc-sa/4.0/.
