Polyneuropathy, Organomegaly, Endocrinopathy, Monoclonal gammopathy and Skin abnormalities (POEMS) Syndrome in a Filipino Nurse Returning to Work Following Bortezomib Treatment: A Case Report
Nadine J. Endaya, Raymond L. Rosales
Oct 2022 DOI 10.35460/2546-1621.2022-0044
Introduction
POEMS syndrome is a rare monoclonal gammopathy. Between 2007 to 2020, among southeast Asian countries which include the Philippines, there are only a few published studies. Overall, the ratio of male and female cases was equal, unlike other countries which reported male predominance. The median age of onset was during the fifth decade of life. Owing to the rarity of this disease, there are no standard treatments, but numerous therapeutic options have been reported. Most of the cases used a combination of melphalan and steroids as first-line treatment, which provided good outcomes.[1] Worldwide, recent studies presented that classic chemotherapy which is an alkylator-based therapy, melphalan, was the least effective with only 20% complete remission rate. The proteasome inhibitor, bortezomib, achieved a 69% complete remission rate described as an improvement in clinical symptoms and serum-free light chain response without the appearance of its adverse effect (eg, neuropathy). Thus, utilization of bortezomib as front-line monotherapy appears to be tolerated and highly effective.[2]
In relapse cases, other treatment options were tested in which the combination of bortezomib and dexamethasone was given together with daratumumab, an immunomodulatory drug[3] or cyclophosphamide. On the other hand, other publications reported the use of bortezomib and dexamethasone only as a first-line regimen for newly diagnosed POEMS syndrome and a majority of these patients include Chinese males in their fifth decade of life. Hematologic response evaluation was based on light chain amyloidosis response criteria and neurological disability assessed through the Overall Neuropathy Limitations Scale (ONLS).[4]
Our aim is to present a case report of a documented Filipino man with POEMS syndrome that achieved significant improvement following bortezomib use. We are unaware of similar reports from the Philippines, specifically alluding to the present case. Also, the implemented treatment is highlighted given that this Filipino nurse was able to return to his work needed during the pandemic times.
Case Presentation

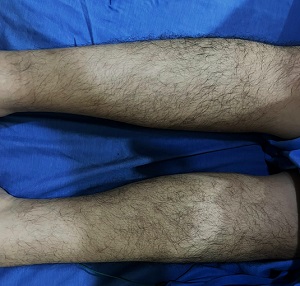
Figure 1. Case of POEMS: Hypertrichosis on bilateral lower legs
A 34-year-old Filipino male was admitted to our institution due to a 3-month history of gradual progressive distal lower extremity weakness described as difficulty during ambulation in which the patient cannot clear his feet off the ground accompanied by minimal erythematous, tender, sharp shooting pain on the left knee and dull aching pain on bilateral legs with a pain scale ranging from 7-9/10. The patient was initially admitted at a hospital in the United Arab Emirates where he was diagnosed and treated as a case of Guillain-Barre syndrome and given intravenous immunoglobulin with no improvements noted. He was also seen by a hematologist-oncologist due to the finding of erythrocytosis and thrombocytosis on complete blood count. It revealed an RBC count of 5.72 x 10^6/uL (reference range 4.5-5.5) and a platelet count of 891 x 10^9/L (reference range 150-410). He was given aspirin 80 mg/tab 1 tab once a day and he underwent phlebotomy but he was lost to follow-up.
In the interim he revealed persistence of lower extremity weakness and pain and hence consulted a neurologist in the Philippines. Upon admission, the patient was wheelchair-bound, awake, oriented to three spheres, and had intact cognitive functions. There were no cranial nerve deficits noted.
The motor examination revealed bilateral lower extremity weakness with more pronounced distal musculature weakness. The bilateral ankle dorsiflexion and plantar flexion had a manual muscle grade of 0-1/5, while bilateral knee flexion and extension had a manual muscle grade of 3+/5 and lastly, bilateral hip abduction, adduction, flexion, and extension had a manual muscle grade of 4+/5. On the other hand, there are good manual muscle grades on all major muscle groups of bilateral upper extremities. There was no spasticity, rigidity, atrophy, fasciculations, or tremor. An impaired position sense on bilateral lower extremities and impaired vibratory sense on bilateral medial malleoli were also noted. The sensation was intact to light touch, pain, and temperature. The patient had generalized areflexia with an ONLS of 5 (arm scale 0/5 and leg scale of 5/7). He required a wheelchair to travel up to 10 meters but was able to stand and walk up to 1 meter with the help of one person. On general physical examination, there was bilateral conjunctival injection and hypertrichosis on bilateral lower legs (Figure 1).
An initial work-up was done which included whole body positron emission tomography with a finding of hypermetabolic focus along the upper paravertebral left lung field/pleural thickening (non-specific) (Figure 2) and irregular/non-homogenous hypermetabolic activity along the bone marrow of the axial and appendicular skeleton, which was also a non-specific finding in the absence of a known malignancy (may be compatible with blood disorder) (Figure 3). A serum-free light chain panel was also done which showed free kappa light chain of 8.10 mg/L (reference interval 7.1-22.49 mg/L), free lambda light chain of 55.3 (reference interval 8.8-23.26 mg/L), and free kappa/lambda light chain ratio of 0.146 (reference interval 0.57-1.25) suspicious for low-level monotype serum-free lambda light chains based on a free lite ratio (Figure 4). An electrophysiologic study revealed chronic bilateral sensorimotor demyelinating polyneuropathy. The serum vascular endothelial growth factor (VEGF) level was also elevated at 3660 pg/ml (cut off value 1040 pg/ml). The bone marrow core biopsy and bone marrow aspirate smear immunohistochemical stain results showed positive for lambda and CD138 supporting the diagnosis of lambda light chain restricted plasma cell myeloma. He was started with melphalan 2 mg/tab 3 tabs TID, prednisone 50 mg/tab 1 tab OD, gabapentin 100 mg/tab 1 tab TID, and aspirin 80 mg/tab 1 tab OD. The patient was discharged stable after five days of hospital stay. The patient noted improvement in symptoms as he reported the ability to lift his foot off the ground only requiring minimal assistance to ambulate within the first three months. No other signs and symptoms were noted such as joint pain, edema, hyperpigmentation, plethora, acrocyanosis, and difficulty breathing.
After six months of regular follow-up and continuous medications, the patient had gynecomastia with a noted breast mass on the right. Excision of the breast mass was done which revealed no atypical plasma cell proliferation present. There was also a recurrence of the weakness of bilateral legs and feet described as a frequent tendency to fall and difficulty ambulating requiring maximal assistance. An elevated lambda chain was noted. Hence, melphalan and prednisone were discontinued. The patient was started on bortezomib 2.3 mg/m2 SC weekly, dexamethasone 4 mg/tab 2 tabs twice a day, and omeprazole 40 mg/tab 1 tab once a day for 12 months.

Figure 2. Case of POEMS: Fluorodeoxyglucose (FDG) uptake along the paravertebral upper lung field along a thickened pleura

Figure 3. Case of POEMS: Inhomogeneous/ irregular Fluorodeoxyglucose (FDG) uptake along the spine and pelvic bone marrow. Inhomogenous uptake in the ribs, right humeral head, left pubic bone, sacrum and right femur
FOLLOW-UP AND OUTCOMES

Figure 4. Serum Electrophoresis (Pre-treatment in a case of POEMS)
The patient showed clinical improvement and hematologic response after administration of bortezomib and dexamethasone. He was able to ambulate independently with support using bilateral ankle-foot orthosis. There was still no reported numbness, joint pains, edema, hyperpigmentation, plethora, acrocyanosis, and difficulty breathing. The manual muscle grade of bilateral hip abduction, adduction, flexion and extension, knee flexion and extension are 5/5 while bilateral ankle dorsiflexion and plantar flexion are 3+/5 and 4+/5, respectively with ONLS of 1 (arm scale 0/5 and leg scale of 1/7). The serum light chain revealed negative for monoclonal serum-free light chains which showed free kappa light chain of 12.7 mg/L (reference interval 7.1-22.49 mg/L), free lambda light chain of 13.4 (reference interval 8.8-23.26 mg/L), and free kappa/lambda light chain ratio of 0.947 (reference interval 0.57-1.25). There was no hypertrichosis and gynecomastia. His ability to walk, climb, and run was affected but his gait did not look abnormal.
Discussion
The diagnosis of POEMS syndrome based on the current Dispenzieri diagnostic criteria requires the presence of both mandatory criteria (polyneuropathy and monoclonal plasma cell-proliferative disorder, almost always lambda restricted), and at least one major and one minor criterion. The major criteria include Castleman disease, sclerotic bone lesions, and VEGF elevation. On the other hand, the minor criteria include organomegaly (splenomegaly, hepatomegaly, or lymphadenopathy), extravascular volume overload (edema, pleural effusion, or ascites), endocrinopathy (adrenal, pituitary, gonadal, parathyroid, thyroid, and pancreatic), skin changes (hyperpigmentation, hypertrichosis, glomeruloid hemangiomata, plethora, acrocyanosis, flushing, and white nails), papilledema, and thrombocytosis/polycythemia. Despite the availability of several criteria, diagnosing POEMS syndrome is challenging due to its multisystemic affection. Detailed history and examinations are essential since it is commonly misdiagnosed as chronic inflammatory demyelinating polyneuropathy.
The pathophysiology of POEMS syndrome remains unknown but high levels of pro-angiogenic, pro-inflammatory cytokines, and growth factors are found to be the hallmark of the disease. The symptoms of polyneuropathy and vascular leak in the form of edema and effusions are due to these factors namely tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL1), interleukin-6 (IL6), and VEGF.[5] Among these factors, VEGF may affect the blood-nerve barrier by increasing permeability, which eventually gives rise to edema and nerve damage due to increased endoneural pressure. Hence, it is considered as the primary factor responsible for a majority of symptoms. Serum VEGF is markedly elevated in this syndrome and that is why one of the treatment options was to target the suppression of VEGF.
Several studies denoted that serum VEGF levels can monitor disease activity and project functional outcome. Ideally, a change in therapeutic strategy should be considered if serum VEGF levels remained elevated after 6 months post treatment. It was found that 6 months are required to determine the effect of treatment because VEGF levels will reach a plateau over 6 months after treatment initiation and decrease afterwards. In relation to its outcome, there will be improvement in clinical symptoms and laboratory parameters at 12 months post treatment.[6] It correlated that normal VEGF levels can prolong remission rates and prevent disease relapses. However, other factors must still be considered and monitored since the suppression of VEGF alone by induction of an anti-VEGF monoclonal antibody, bevacizumab, does not appear to be fully effective in preventing relapses, especially in advanced cases.[7] This was also seen in patients subjected only to corticosteroids for treatment.[8]
There is no standard first-line therapy for newly-diagnosed POEMS syndrome but current treatment approaches provide a good prognosis based only on one randomized controlled study that has been completed. Treatment for newly diagnosed POEMS syndrome depends on the extension of disease. The current guidelines suggest that curative doses of radiation (40–50 Gy) are recommended in patients with isolated bone lesion without bone marrow clonal plasma cell involvement while systemic therapy is recommended in patients with disseminated disease (more bone lesions and/or bone marrow plasmacytosis).[9] On the other hand, there are no established standards of care management in relapse cases of POEMS, though there are few cases that showed that bortezomib, a proteasome inhibitor, was effective and safe.
Bortezomib targets the ubiquitin-proteasome pathway. It decreases VEGF expression by downregulating several angiogenic cytokines, such as VEGFs, FGF-2, IGF-1, interleukins, and the expression of angiopoietins. Its antiangiogenic action is primarily due to the prevention of degradation of nuclear factor kappa-B kinase complex (INK) inhibitor and resultant block of nuclear factor kappa light chain enhancer of activated B cells (NF-κB) activity.[10]
In a study conducted in Shanghai Changzheng Hospital in China from December 2014 to May 2016, VEGF levels were determined before and after bortezomib treatment. Normal values of VEGF were defined as below 600 pg/ml and the response rate was determined after induction of bortezomib. The response rate was measured in terms of reduction of serum VEGF level. A majority of subjects showed complete response which was defined as a reduction of serum VEGF level to normal values. The reduction of serum VEGF levels coincided with improvement of the subject’s ONLS from a score of 5 at the time of diagnosis to 3.[4] Thus, hematologic response evaluation was important in the assessment, including serum light chain levels and serum VEGF level.
Conclusion
We have documented a case of a Filipino male with confirmed POEMS syndrome or Crow-Fukase syndrome. The combination of bortezomib 2.3 mg/m2 SC weekly and dexamethasone 4 mg/tab 2 tabs a day for 12 months are proven to be safe and responsive in improving symptoms in relapsed cases during early adulthood. The serum VEGF levels post-treatment must still be accounted to predict remission rates and plan future therapeutic goals and strategies.
- Prado MB, Adiao KJ. Clinical features of POEMS syndrome in Southeast Asia: A literature-based study. SN Compr. Clin. Med. 2022;4(48). [cited 2022 June 18]. Available from: https://doi.org/10.1007/s42399-022-01130-3.
- Jurczyszyn A, Castillo JJ, Olszewska-Szopa M, Kumar L, Thibaud S, Richter J, et al. POEMS syndrome: Real world experience in diagnosis and systemic therapy – 108 patients multicenter analysis. Clinical Lymphoma, Myeloma & Leukemia. 2022; 22(5):279–304. [cited 2022 June 18]. Available from: https://doi.org/10.1016/j.clml.2021.10.007.
- Khwaja J, Keh R, Smyth D, Lunn MP, D’Sa S, Sive J. Daratumumab-bortezomib-dexamethasone use in relapsed POEMS syndrome. EJHaem [Internet]. 2022;3(3):1021–4. Available from: http://dx.doi.org/10.1002/jha2.492.
- He H, Fu W, Du J, Jiang H, Hou J. Successful treatment of newly diagnosed POEMS syndrome with reduced-dose bortezomib based regimen. Br J Haematol [Internet]. 2018;181(1):126–8. Available from: http://dx.doi.org/10.1111/bjh.14497.
- Nozza A. Poems Syndrome: An update. Mediterr J Hematol Infect Dis. 2017;9(1). 10.4084/MJHID.2017.051. [cited 2022 June 27].
- Misawa S, Sato Y, Katayama K, Hanaoka H, Sawai S, Beppu M, et al. Vascular endothelial growth factor as a predictive marker for POEMS syndrome treatment response: retrospective cohort study. BMJ Open [Internet]. 2015;5(11):e009157. Available from: http://dx.doi.org/10.1136/bmjopen-2015-009157
- Straume O, Bergheim J, Ernst P. Bevacizumab therapy for POEMS syndrome. Blood. 2006;107(12):4972–4. doi:10.1182/blood-2005-12-5045
- Ji ZF, Zhang DY, Weng SQ, Shen XZ, Liu HY, Dong L. POEMS syndrome: A report of 14 cases and review of the literature. ISRN Gastroenterol [Internet]. 2012;2012:584287. Available from: http://dx.doi.org/10.5402/2012/584287
- Dispenzieri A. POEMS syndrome: 2021 Update on diagnosis, risk- stratification and management. Annual Clinical Updates in Hematological Malignancies. 2021. Available from: https://doi.org/10.1002/ajh.26240.
- Ria R, Melaccio A, Racanelli V, Vacca A. Anti-VEGF drugs in the treatment of multiple myeloma patients. J Clin Med [Internet]. 2020;9(6):1765. Available from: http://dx.doi.org/10.3390/jcm9061765.
![]() Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0
International License, which permits use, share — copy and redistribute the material in any medium or format,
adapt — remix, transform, and build upon the material, as long as you give appropriate credit,
provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner,
but not in any way that suggests the licensor endorses you or your use. You may not use the material for
commercial purposes. If you remix, transform, or build upon the material, you must distribute your
contributions under the same license as the original. You may not apply legal terms or technological
measures that legally restrict others from doing anything the license permits. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons
license and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this license,
visit https://creativecommons.org/licenses/by-nc-sa/4.0/.
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike 4.0
International License, which permits use, share — copy and redistribute the material in any medium or format,
adapt — remix, transform, and build upon the material, as long as you give appropriate credit,
provide a link to the license, and indicate if changes were made. You may do so in any reasonable manner,
but not in any way that suggests the licensor endorses you or your use. You may not use the material for
commercial purposes. If you remix, transform, or build upon the material, you must distribute your
contributions under the same license as the original. You may not apply legal terms or technological
measures that legally restrict others from doing anything the license permits. The images or other
third party material in this article are included in the article’s Creative Commons license, unless indicated
otherwise in a credit line to the material. If material is not included in the article’s Creative Commons
license and your intended use is not permitted by statutory regulation or exceeds the permitted use,
you will need to obtain permission directly from the copyright holder. To view a copy of this license,
visit https://creativecommons.org/licenses/by-nc-sa/4.0/.
